News
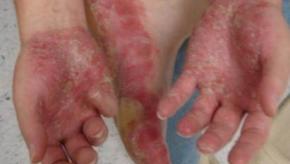
FDA Panel Recommends Brodalumab Approval in Psoriasis
Valeant Pharmaceuticals International's experimental drug to treat psoriasis should be approved as long as certain measures are put in place to mitigate the risk of suicide, an advisory committee to the U.S. Food and Drug Administration concluded on Tuesday.Epigenetic Changes to Inflammasome Found in Autoinflammatory Syndromes
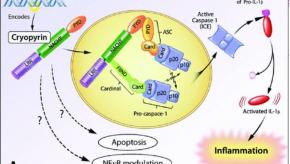
The gene mutations underlying inflammasome activity have been well described, but there appears to be variable penetrance among patients with the same gene mutation, suggesting additional mechanisms may influence disease expression.Little Evidence Favoring the Health Benefits of Vitamin D
Vitamin D certainly plays a pivotal role in bone health and immune function.
DEXA Still Needed While on Bisphosphonates
Reuters reports that a study of 6629 Canadian women with osteoporosis taking bisphosphonates found that nearly one in five had a decrease in bone density while on medication. (Citation source: http://buff.ly/2afb7nj)
Prevalence of Spondyloarthropathy in Fibromyalgia
With the introduction of ASAS criteria for the diagnosis of axial and peripheral spondyloarthropathy, there has been a debate about whether patients with fibromyalgia (FM) could be mistaken for a spondyloarthropathy, or vice versa.
SAPHO Syndrome Revisted
SAPHO is an acronym that describes a constellation of findings (synovitis, acne, pustulosis, hyperostosis, and osteitis) that defines this rare syndrome.
Will Patent Extensions Delay Biosimilar Arrivals?
In the last 6 months, the FDA Arthritis Advisory Committee has recommended three biosimilars (CT-P13, GP-2015, ABP-501) for approval, with one achieving FDA approval Inflectra/CT-P13) and the other two pending a probable approval in the months to come.
ORBIT Study Shows Rituximab is Non-Inferior to TNF Inhibitors in Biologic Naive Patients
Porter and colleagues have reported that both rituximab (RTX) and tumour necrosis factor inhibitors (TNFi) are equally and highly effective in early, active, biologic-naive rheumatoid arthritis patients.
Increaed GI Perforations with Tocilizumab Seen in German Registry Analysis
The issue of lower intestinal tract perforation (LIP) is no novelty to rheumatologists since the RA treatment paradigm shifted from use of NSAIDs (the most common cause of upper GI tract perforations) to steroids and non-steroidal DMARDs.
RheumNow Week in Review – 15 July 2016
Dr. Jack Cush reviews highlights from this week's rheumatology news on RheumNow.com.
Sandoz Etanercept Biosimilar Voted for Approval by FDA Arthritis Advisory Committee
The Food and Drug Administration’s Arthritis Advisory Committee (AAC) yesterday recommended that the Sandoz etanercept (Enbrel) biosimilar (GP-2015) be approved for use in the United States.