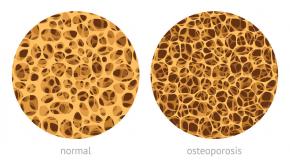
FDA Panel Backs Approval of Romosuzumab for Osteoporosis
The US Food and Drug Administration’s advisory committee on Bone, Reproductive and Urologic Drugs voted Wednesday in favor of approving Amgen’s osteoporosis anti-sclerostin drug, romosozumab (Evenity) for use women with postmenopausal osteoporosis.