
Richard Conway RichardPAConway
3 years 6 months ago
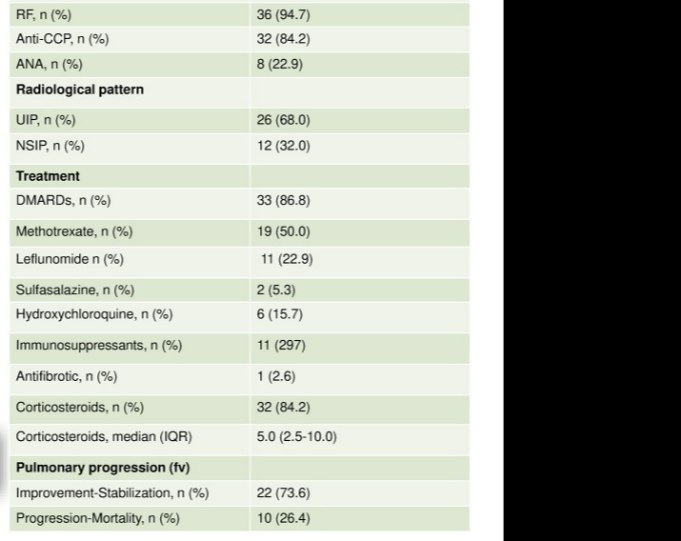
Redondo-Rodriguez et al. Abatacept in RA-ILD. Prospective study 38 patients. 73.6% improved/stablised with abatacept at median 17 month follow-up @RheumNow #EULAR2022 POS0654 https://t.co/D2du0hAXIy

Aurelie Najm AurelieRheumo
3 years 6 months ago
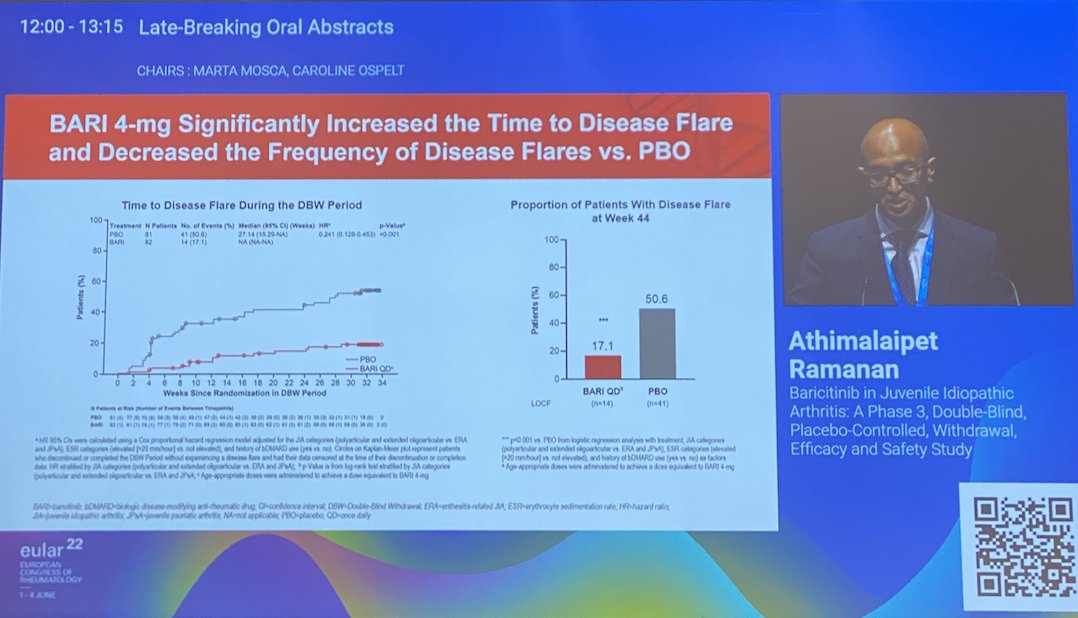
JUVE-BASIS Baricitinib in JIA
Phase 3 RCT wk12
Cs or BioDMARDs IR
⚡️Time to disease flare HR 0.24
⚡️JIA-ACR50% 63%
⚡️% flares 17% vs. 50% in PBO
Safety profile similar than adults
@RheumNow
#EULAR2022 LB0002 https://t.co/dX3DpB4QHT

David Liew drdavidliew
3 years 6 months ago
@EricFMorand up first in late-breakings
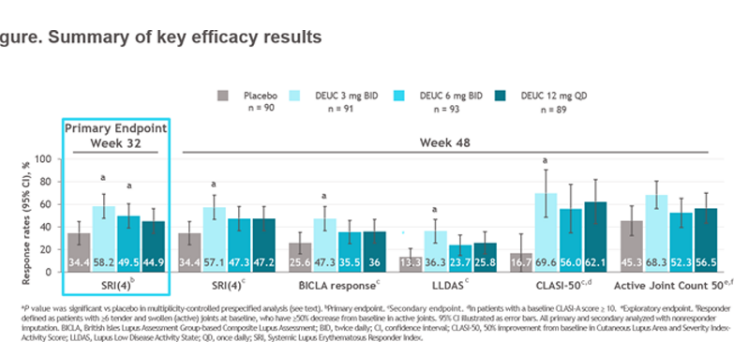
PAISLEY: deucravacitinib (TYK2i) ph2 in SLE - add-on, steroid wean
Good outcomes:
primary: SRI(4) at 32w
skin/joint/LLDAS & dsDNA/C4
Safety good (like PsO/PsA) but higher dose less so
Watch for: other clinical, ph3
#EULAR2022 @RheumNow https://t.co/XOQWuiHkTx

Md Yuzaiful Md Yusof Yuz6Yusof
3 years 6 months ago
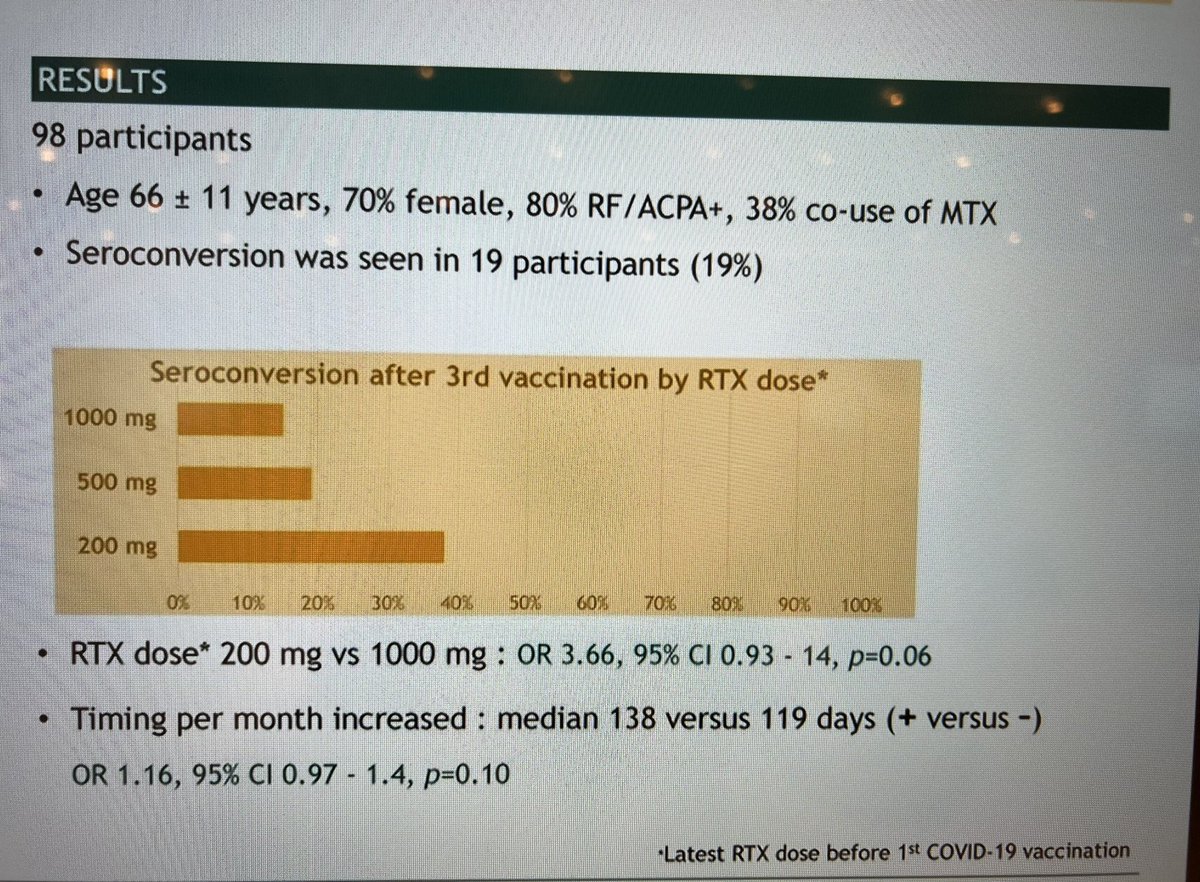
#POS1207 #EULAR2022 In N=98 RA patients with poor antibody response to rituximab after two doses, 19% responded after 3rd dose. Associated with longer Time of vaccine from last RTX & interestingly ultra dose 200mg used in the Netherlands @RheumNow https://t.co/TTXXtOU6vp

Eric Dein ericdeinmd
3 years 6 months ago
#EULAR2022 LB0001
Bimekizumab (IL-17F and IL-17A) in bionaive PsA
BE OPTIMAL phase 3 trial, 24 weeks
⭐️Met Primary endpt - ACR50
⭐️Met other ACR and PASI endpts
⭐️Safety: higher fungal infections
@RheumNow https://t.co/x8u6B93FNl

Dr. Antoni Chan synovialjoints
3 years 6 months ago
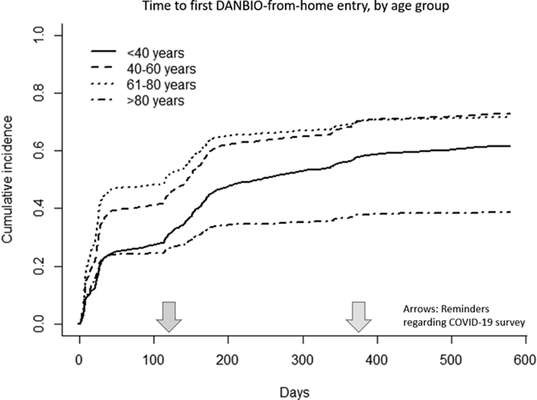
Who remotely enters PROs online? Glintborg et al from the Danbio Registry showed that patients who used the system were mainly 40-80 years of age, on biologics and had lower HAQ score. Disease duration and diagnosis had no impact, reminders helped #EULAR2022 @RheumNow POS0373 https://t.co/voCfGiJgwm

Richard Conway RichardPAConway
3 years 6 months ago
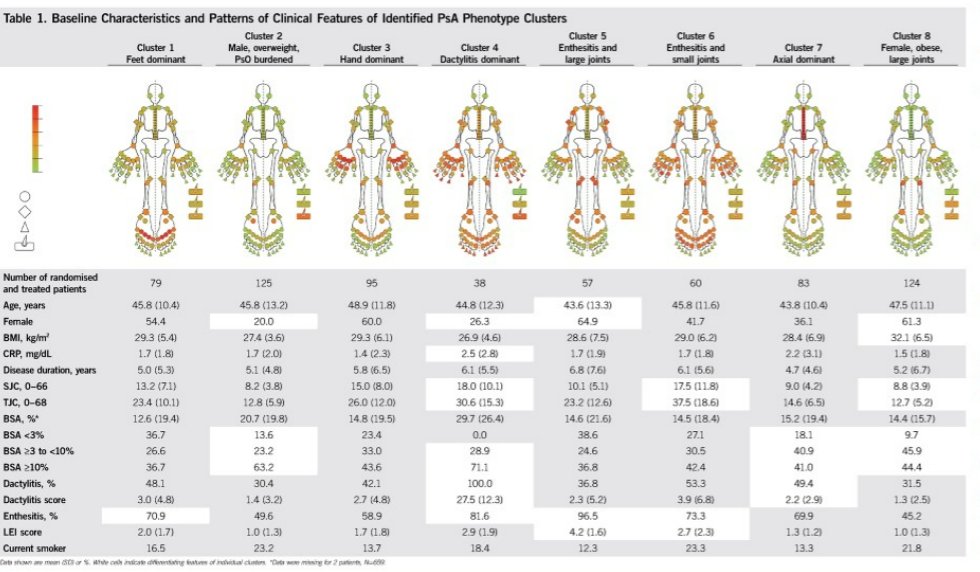
Fascinating! Richette et al. Machine learning identifies 8 clusters of PsA phenotypes. Treatment responses seem to differ, being better in cluster 2 and worse in cluster 5 and 6 (at least for IL23i guselkumab). @RheumNow #EULAR2022 POS1055 https://t.co/QIh2SbJKpa

Richard Conway RichardPAConway
3 years 6 months ago
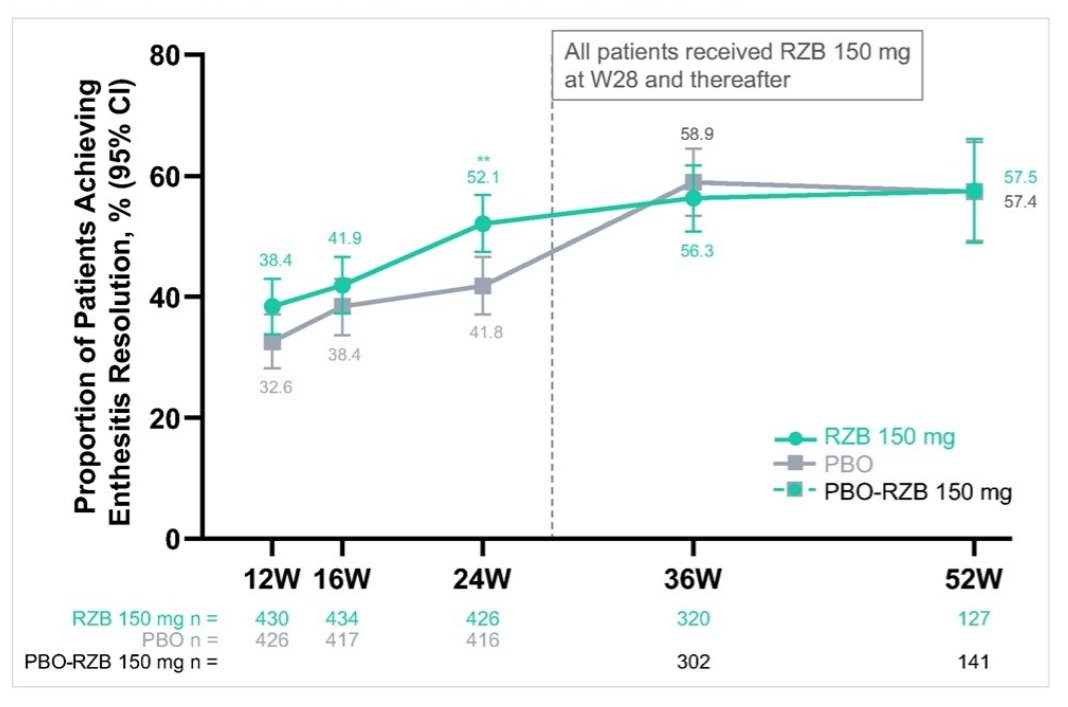
Magrey et al. IL23i Risankizumab improves enthesitis in PsA @RheumNow #EULAR2022 POS1057 https://t.co/BjuYj6xUdZ

TheDaoIndex KDAO2011
3 years 6 months ago
Wow! Phase 2 #deucravacitinib data for #SLE met primary & secondary endpoints: SRI(4) response, BICLA, LLDAS, CLASI-50. AEs include: skin related events and UTIs but no increase in SIE, HZ, MACE/VTE @bmsnews LB0004 #EULAR022 @rheumnow https://t.co/bsKI0mixVT

Md Yuzaiful Md Yusof Yuz6Yusof
3 years 6 months ago
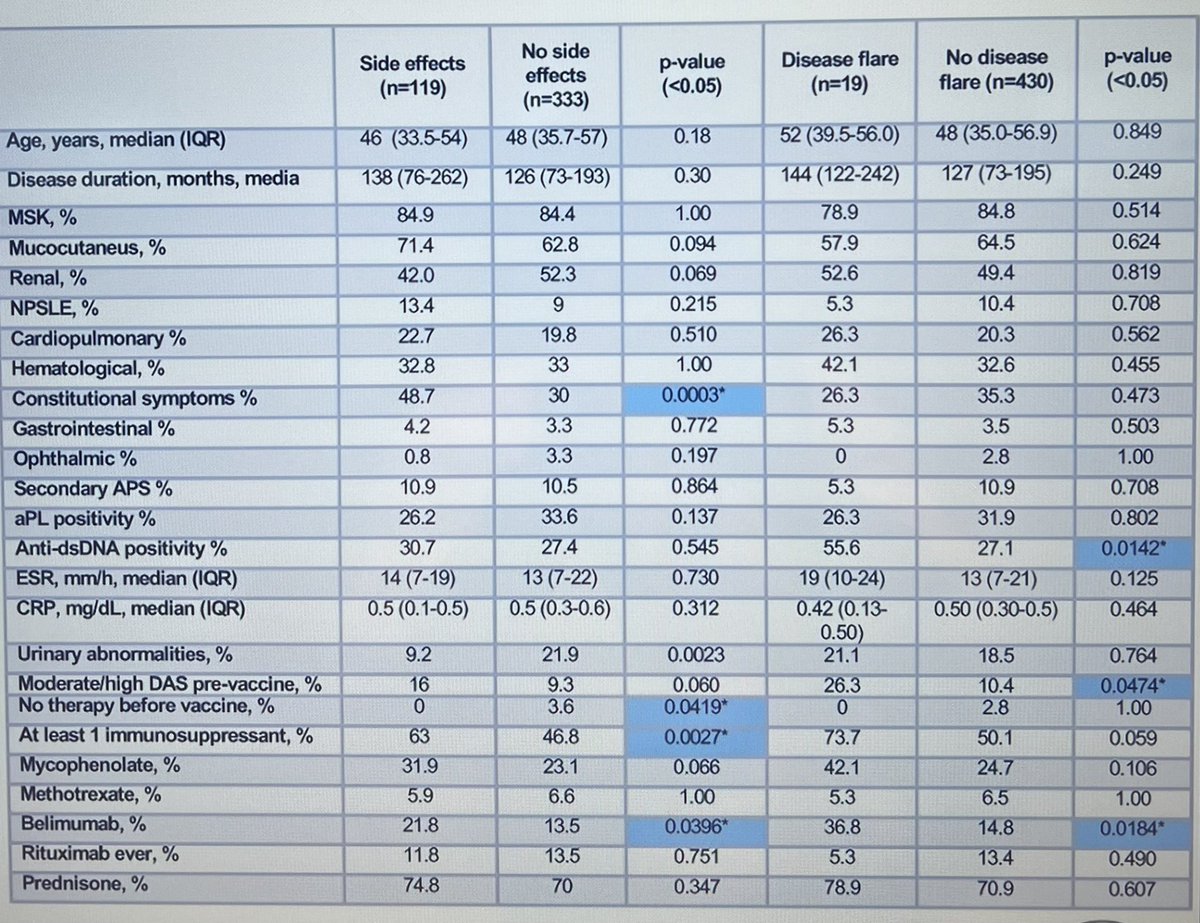
#POS1236 #EULAR2022 More data to justify safety of #COVID vaccination to our #lupus patients. A study in Italy (>450 pts) found 1/4 had side effects (mostly mild) and 4% had a flare. These side effects were more common in those on >1 DMARDS and Belimumab @RheumNow https://t.co/mNS6rQfsSq

David Liew drdavidliew
3 years 6 months ago
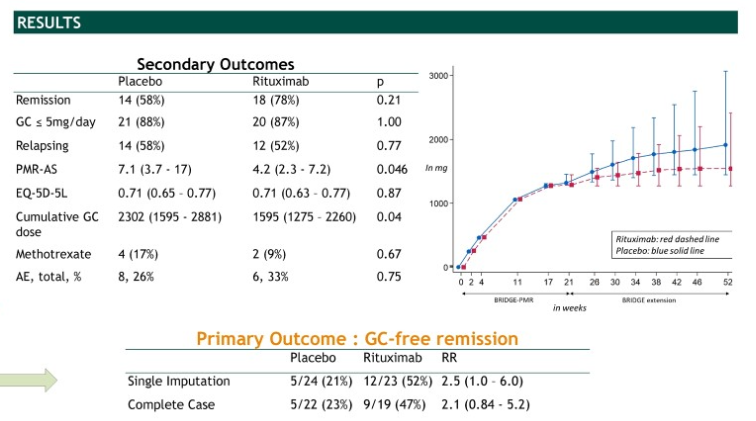
BRIDGE-PMR (rituximab in PMR) routine care extension to 52w
(in #PMRGCAJC one big qu asked was ?sustained benefit)
sustained benefit (RR 2.5 GC-free remission)
steroid-sparing (PNL 1.6g v 2.3g)
& ritux ?cheap enough now
but COVID calculus difficult
POS0269 #EULAR2022 @RheumNow https://t.co/Ln2ArclKO8 https://t.co/OtCwdjIPeL

Dr. Antoni Chan synovialjoints
3 years 6 months ago
What guides treatment decisions in AxSpA? Bolt et al showed patients assessed in clinical remission, 1/3 had BASDAI >3.5. Treatment unchanged as low disease activity, awaiting effects of current Rx, complaints not related to axSpA, patient preference #EULAR2022 @RheumNow POS0303

Dr. Antoni Chan synovialjoints
3 years 6 months ago
Treatment of active SpA improves depressive symptoms in SpA with HADS-D score reducing. This was greatest for TNFi followed by NSAIDs and csDMARDs. TNFi reduced the proportion of patients (48% to 29%) with depressive symptoms at follow up Webers et al #EULAR2022 @RheumNow POS0304

Richard Conway RichardPAConway
3 years 6 months ago
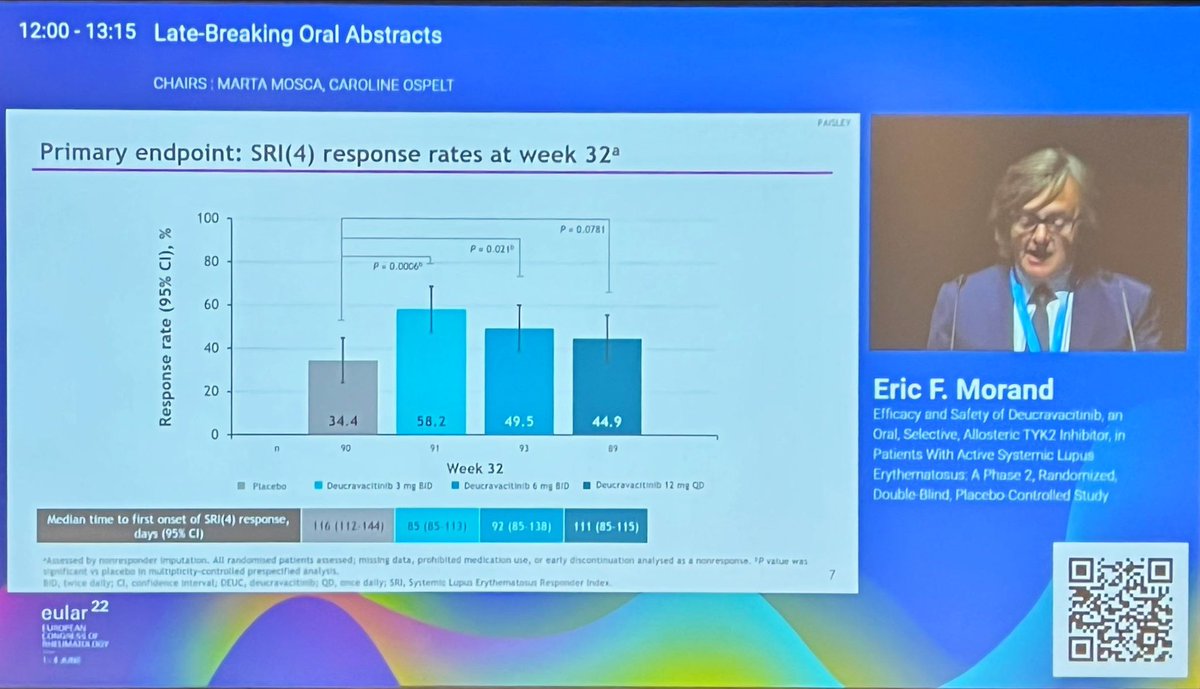
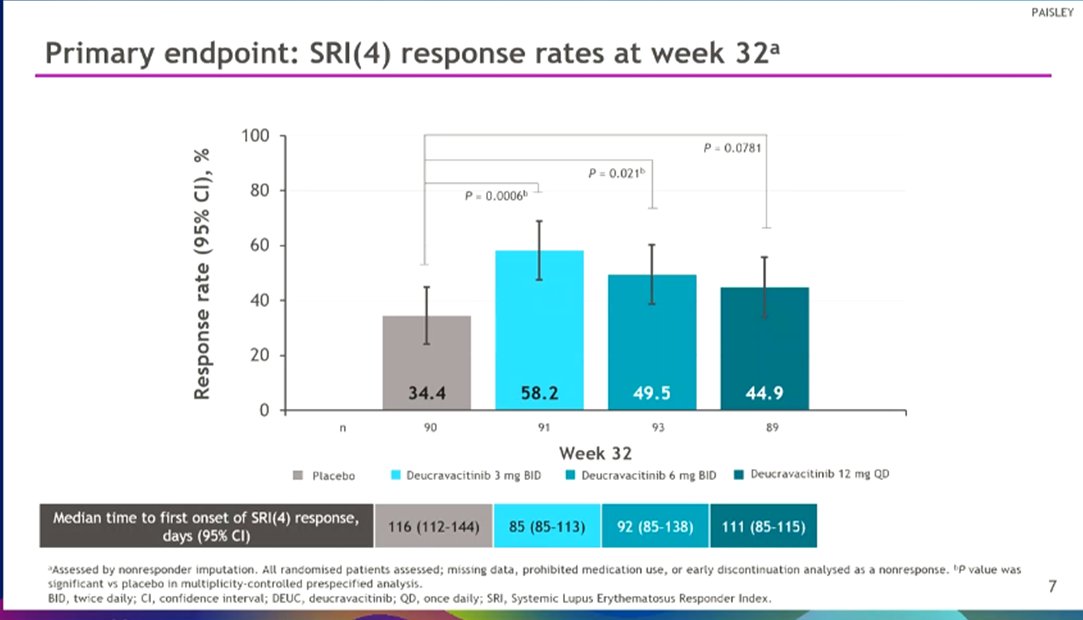
Morand et al Tyk2i deucravacitinib in SLE. 48-week phase 2 RCT. SRI(4) PBO: 34.4%; DEUC 3 mg BID: 58.2%, P =0.0006; DEUC 6 mg BID: 49.5%, P =0.021; DEUC 12 mg QD: 44.9%, P =0.078. BICLA, LLDAS, CLASI-50, active joint count also+ @RheumNow #EULAR2022 LB0004 https://t.co/MBPc9xQFvG https://t.co/n3GUwl0RtD

Aurelie Najm AurelieRheumo
3 years 6 months ago
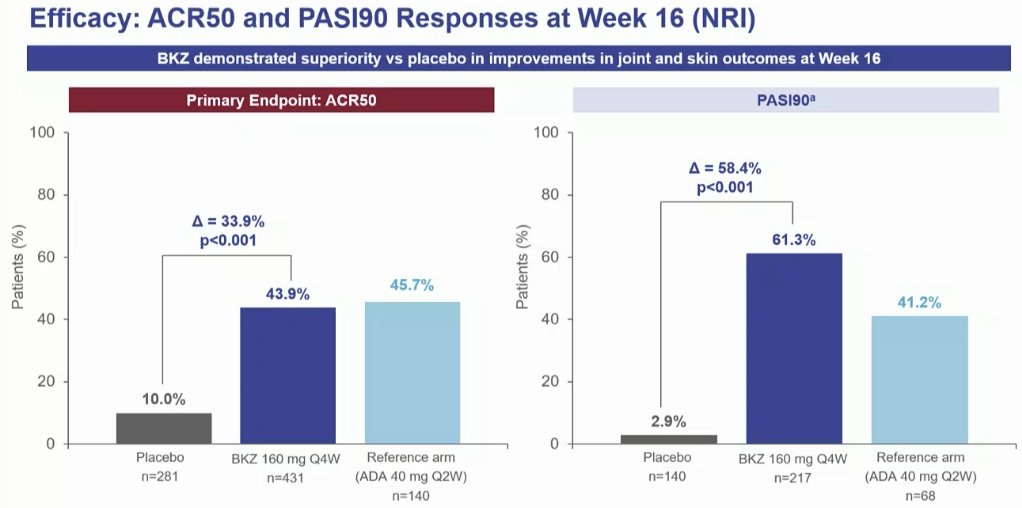
BE OPTIMAL Phase 3 RCT
Bimekizumab dual IL17A & F inhibitor in bionaive PsA
⭐️ACR50 wk 16 43.9% vs 10% PBO
⭐️PASI 90 wk 16 61% vs. 3% PBO
⭐️separation from wk 4
Safety: fungal injections BKZ > ADA
@RheumNow
LB0001 #EULAR2022 https://t.co/7ZwV1XDSNb


















 Poster Hall
Poster Hall